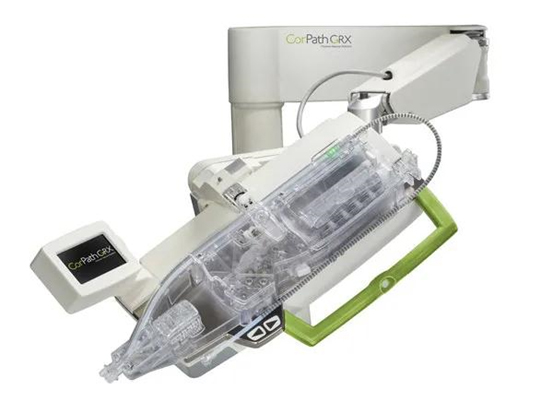
On July 30, 2021, Corindus CorPath GRX Robotic PCI System (CorPath GRX) of Siemens Healthineers was approved to enter the innovation pathway by the CMDE. Siemens Healthineers now became the first foreign-funded medical imaging equipment manufacturer who has entered the innovation pathway.
With the beneficial policies in Boao Lecheng Pilot Zone (Hainan, China), Siemens Healthineers completed its first robot-assisted coronary intervention operation in Hainan Boao Super Hospital several months ago, which attracted extensive attention in the industry.
CorPath GRX is designed to protect and assist interventional cardiologists in complex PCI, which can eliminate radiation exposure for primary operators and patients during surgery and help to standardize procedures. Compared with traditional manual interventional surgery, CorPath GRX is a leading medical technology with fundamental improvement.
The Special Review Procedure for Innovative Medical Devices revised by NMPA, aims to encourage the R&D and innovation of medical devices and take new technologies of medical devices into practice. As a fast track, the Special Review Procedure for Innovative Medical Devices[1] was implemented on December 1, 2018. After the medical devices enter the innovation pathway, NMPA will give priority to the review and approval of innovative medical devices, which will expedite the registration process for manufacturer accordingly. IMD has extensive experience with all kinds of preferential policies in China and has helped with the registration of many innovative medical devices. If you are interest in the China market, please feel free to contact us (info@inspirativemed.com) for information about fast track in Hainan Boao Lecheng International Medical Tourism Pilot Zone.








